
Satya Dubey
Cranfield University, UK
Title: Catalytic Synthesis of Environmental friendly Polymer Poly Lactic Acid via Continuous Reactive Extrusion: Recent Trends
Biography
Biography: Satya Dubey
Abstract
The disposal of large amount of polymer waste is one of the major challenges of this century. Use of bio-degradable polymers obtained from sustainable sources presents a solution to this problem. Poly lactic acid (PLA), a bio-degradable polymer,can be synthesized from sustainable sources as corn, starch,sugarcane and chips.Ring opening polymerization (ROP) of lactide monomer using metal/bimetallic catalyst (Sn, Zn or Al) is the preferred method for synthesis of PLA. However, the PLA synthesized using such catalysts may contain trace elements of the catalyst. These catalyst traces are known carcinogens and as such should be (ideally) eliminated from the process. Continuous reactive extrusion of lactide monomer (using the suitable reaction input has the potential to increase the throughput, and this route has been explored in the literature. In this work, reactive extrusion experiments using stannous octoate Sn(Oct)2 and tri-phenyl phosphine (PPh)3, were considered to perform ROP of lactide monomer using the microwave as an alternative energy (AE) source for activating and/or boosting the polymerization (Figure 1). Implementation of a microwave generator in a section of the extruder is one of the novelties of this research. A simulation model of ROP of PLA was formulated to estimate the impact of reaction kinetic and AE source on the polymerization process. Ludovic® software was used for the simulation of continuous reactive extrusion of the process. Experimental and simulated results were compared for the validation of the methodology. This work also highlights the advantages and drawbacks of most conventional metal catalysts, the effect of alternative energies on reaction mechanism, and safe and efficient production of PLA.
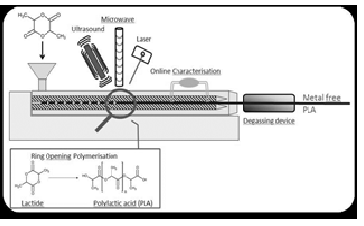
Figure 1 Polymerization overview
Recent Publications:
- Satya P Dubey1, Hrushikesh A Abhyankar, Veronica Marchante, James L Brighton, Kim Blackburn, Clive Temple, Björn Bergmann, Giang Trinh, Chantal David, “Modelling and Validation of Synthesis of Poly Lactic Acid using Alternative Energy Source through Continuous Reactive Extrusion Process” Polymers (Vol:8, 164; doi:10.3390/polym8040164 2016
- Satya P Dubey1, Hrushikesh A Abhyankar, Veronica Marchante, James L Brighton, Björn Bergmann, Giang Trinh, Chantal David, “Microwave Energy assisted Synthesis of Poly Lactic Acid through Continuous Reactive Extruder: Reaction kinetics modelling approach” (RSC Advances, 2017, 7, 18529 – 18538. DOI: 10.1039/c6ra26514f.)
- Satya P Dubey1, Hrushikesh A Abhyankar, Veronica Marchante, James L Brighton, Kim Blackburn, “Review on catalytic progress on poly lactic acid formation through ring opening polymerization” (International Research Journal of Pure and Applied Chemistry)”. 2016 – Vol: 12, issue 3, IRJPAC/26179
- Satya P Dubey1, Hrushikesh A Abhyankar, Veronica Marchante, James L Brighton, Björn Bergmann, “Mathematical Modeling for Continuous Reactive Extrusion of Poly Lactic Acid formation by Ring Opening Polymerization Considering Metal/Organic Catalyst and Alternative Energies” International Journal of Chemical, Nuclear, Materials and Metallurgical Engineering-( Vol:9, No:2, 2015)
- A. Ojha, N. Vyas, and Satya P Dubey, “Gas phase structural stability of neutral and zwitterionic forms of alanine in presence of (H2O)n=1–7 clusters: A density functional theory study,” Comput. Theor. Chem., vol. 1002, pp. 16–23, Dec. 2012.

