
Philippe Lainé
Université Paris Diderot, France
Title: From single-electron processes to multielectron handling and storage at the molecular level: Designing super-electrophores for the next generation of prototypes of Tranducers for man-made photosynthesis?
Biography
Biography: Philippe Lainé
Abstract
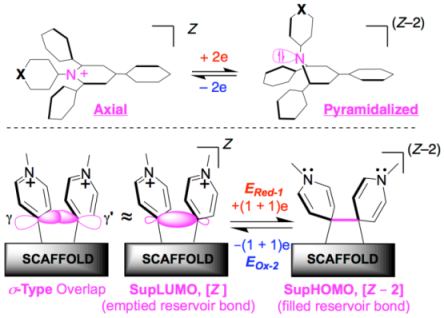
As part of our continuing research program devoted to artificial photosynthesis, which relies on multi-photon and multi-electron processes, we have recently revisited the physical chemistry, and especially the electrochemistry of pyridinium derivatives as multi-electron acceptors. Here we report on the design and rich electrochemistry of two classes of super-electrophores that share the uncommon feature of being able to undergo a two-electron reduction in a single step.The functioning of these superelectrophores relies on the intriguing phenomenon of potential inversion which can be implemented in different ways that actually correspond to two different electrochemical paradigms. On the one hand, there are polyaryl-substituted pyridiniums referred to as branched Expanded Pyridiniums (EPs), that are multifunctional platforms featuring good electrophoric properties and also effective chromophoric and luminophoric activities. On the other hand, there are specifically assembled multi-electrophoric compounds, referred to as Structronic Assemblies (SAs), characterized by their electrochemical hysteresis, that allow the storage of electrons in the form chemical bonds used as electron reservoirs. Special emphasis is herein placed on the rationalization of electrophoric properties and the mechanisms that explain the unusual electrochemical behavior of these two classes (EPs & SAs) of super-electrophores. These studies combine various experimental methods (crystallography, NMR, electrochemistry as well as in situ UV-vis. and IR spectroelectrochemistry) with theoretical modeling. Finally, the manner by which these types of super-electrophores (EPs & SAs) could be used within the framework of research devoted to man-made photosynthesis will be evoked.As part of our continuing research program devoted to artificial photosynthesis, which relies on multi-photon and multi-electron processes, we have recently revisited the physical chemistry, and especially the electrochemistry of pyridinium derivatives as multi-electron acceptors. Here we report on the design and rich electrochemistry of two classes of super-electrophores that share the uncommon feature of being able to undergo a two-electron reduction in a single step.The functioning of these superelectrophores relies on the intriguing phenomenon of potential inversion which can be implemented in different ways that actually correspond to two different electrochemical paradigms. On the one hand, there are polyaryl-substituted pyridiniums referred to as branched Expanded Pyridiniums (EPs), that are multifunctional platforms featuring good electrophoric properties and also effective chromophoric and luminophoric activities. On the other hand, there are specifically assembled multi-electrophoric compounds, referred to as Structronic Assemblies (SAs), characterized by their electrochemical hysteresis, that allow the storage of electrons in the form chemical bonds used as electron reservoirs. Special emphasis is herein placed on the rationalization of electrophoric properties and the mechanisms that explain the unusual electrochemical behavior of these two classes (EPs & SAs) of super-electrophores. These studies combine various experimental methods (crystallography, NMR, electrochemistry as well as in situ UV-vis. and IR spectroelectrochemistry) with theoretical modeling. Finally, the manner by which these types of super-electrophores (EPs & SAs) could be used within the framework of research devoted to man-made photosynthesis will be evoked.
Recent Publications:
- Arrigo A, Santoro A, Puntoriero F, Lainé PP, Campagna S (2015) Photoinduced electron transfer in donor-bridge-acceptor assemblies: the case of Os(II)-bis(terpyridine)-(bi)pyridinium dyads. Coord. Chem. Rev., 304–305: 109–116.
- (a) Fortage J, Tuyèras F, Ochsenbein P, Puntoriero F, Nastasi F, Campagna S, Griveau S, Bedioui F, Ciofini I, Lainé, PP (2010) Expanded Pyridiniums: Bis-cyclization of Branched Pyridiniums into Their Fused Polycyclic and Positively Charged Derivatives – Assessing the Impact of Pericondensation on Structural, Electrochemical, Electronic and Photophysical Features. Chem. Eur. J., 16: 11047–11063. (b) Fortage J, Peltier C, Nastasi F, Puntoriero F, Tuyèras F, Griveau S, Bedioui F, Adamo C, Ciofini I, Campagna S, Lainé PP (2010) Designing Multifunctional Expanded Pyridiniums: Properties of Branched and Fused Head-to-Tail Bipyridiniums. J. Am. Chem. Soc., 132: 16700–16713.
- Fortage J, Peltier C, Perruchot C, Takemoto Y, Teki Y, Bedioui F, Marvaud V, Dupeyre G, Pospísil L, Adamo C, Hromadová M, Ciofini I, Lainé PP (2012) Single-Step versus Stepwise Two-electron Reduction of Polyarylpyridiniums: Insights from the Steric Switching of Redox Potential Compression. J. Am. Chem. Soc., 134: 2691–2705.
- Lachmanová Š, Dupeyre G, Tarábek J, Ochsenbein P, Perruchot C, Ciofini I, Hromadová M, Pospíšil L, Lainé PP (2015) Kinetics of Multielectron Transfers and Redox-Induced Structural Changes in N-Aryl-Expanded Pyridiniums: Establishing Their Unusual, Versatile Electrophoric Activity. J. Am. Chem. Soc., 137: 11349–11364.

