
Tuanli Yao
Shaanxi University of Science and Technology, China
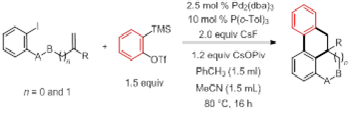
Title: Palladium-Catalyzed Domino Heck/Aryne Carbopalladation/C-H Functionalization: Synthesis of Heterocycle-Fused 9,10-Dihydrophenanthrenes
Biography
Biography: Tuanli Yao
Abstract
Arynes generated from the corresponding o-(trimethylsilyl) aryl triflates have emerged as powerful synthons in organic synthesis.Aryne annulation reactions with intramolecular C-H functionalization can be a powerful method for the synthesis of polycyclic compounds.We developed a novel palladium-catalyzed domino Heck/aryne carbopalladation/C-H functionalization reaction using in situ generated arynes, in which three new C-C bonds and a carbon quaternary center are formed. This methodology affords moderate to excellent yields of heterocycle-fused 9, 10-dihydrophenanthrenes.
Recent Publications:
- Yao, T.; Liu, D.; Zhang, C. “Palladium-catalyzed domino Heck/intermol ecular cross-coupling: efficient synthesis of 4-alkylated isoquinoline derivatives” Chem. Commun. 2017, 53, 2386.
- Yao, T.; Dan, H. “Palladium-Catalyzed Domino Heck/Aryne Carbopalladation/C-H Functionalization:Synthesis of Heterocycle-Fused 9,10-Dihydrophenanthrenes”, Org. Lett. 2017, 19, 842
- Yao, T.; Zhang, H.; Zhao, Y. “Synthesis of 9,10-Phenanthrenes via Palladium-Catalyzed Aryne Annulation by o-Halostyrenes and Formal Synthesis of (±)-Tylophorine”, Org. Lett. 2016, 18, 2532.
- Yao, T. “Facile N-arylation of amidines and N,N-disubstituted amidines”, Tetrahedron Lett. 2015, 56, 4623.
- Schroeder, C. E.; Yao, T.; Sotsky, J. B.; Smith, R. A.; Roy, S.; Chu, Y. K.; Guo, H.; Tower, N. A.; Noah, J. W.; McKellip, S.; Sosa, M.; Rasmussen, L.; Smith, L. H.; White, E. L.; AubeÌ, J.; Jonsson, C. B.; Chung, D.-H.; Golden, J. E. “Development of (E)-2-((1,4-dimethylpi-perazin-2-ylidene)amino)-5-nitro-N-phenylbenzamide, ML336: novel2-amidinophenylbenzamides as potent inhibitors of Venezuelan equine encephalitis virus”. J. Med. Chem. 2014, 57, 8608..

